| Zurück zur Homepage | (Veröffentlicht mit
freundlicher Genehmigung von Dr.
W-E Lönnig) |
Research Signpost
37/661 (2), Fort
P.O., Trivandrum-695 023, Kerala, India
(Recent Res. Devel. Genet. Breeding,
2 (2005): 45-70 ISBN: 81-308-0007-1 )
Lönnig, W.-E.:
Mutation breeding, evolution,
and the law of recurrent variation.
In: Recent Research Developments
in Genetics and Breeding
(G. Pandalai, Managing Editor), Vol. 2 ,
45-70
(2005).
Wolf-Ekkehard Lönnig
Max-Planck-Institut for Plant Breeding Research, Carl-von-Linné-weg
10
50829 Cologne, Federal Republic of Germany
Correspondence/Reprint request: Dr. Wolf-Ekkehard Lönnig, Max-Planck-Institut for Plant Breeding Research Carl-von-Linné-weg 10, 50829 Cologne, Federal Republic of Germany. E-mail: loennig@mpiz-koeln.mpg.de
Abstract:
Abstract In the present paper the history of the rise and fall of mutation breeding as an autonomous branch of breeding research is documentedas well as its positive side effects for plant breeding and biology in general. Perhaps the most important generalization on the basis of the total outcome of mutation breeding will be termed “the law of recurrent variation”. It states that “treating homozygous lines with mutagenic agents generates large, but clearly finite, spectra of mutants. This consistently occurs when the experiments are carried out on a scale adequate to isolate the potential of alleles causing phenotypic and functional deviations(saturation mutagenesis). However, due to almost invisible residual effects of changes in redundant sequences and/or of further chromosome rearrangements, the corresponding saturation curve is asymptotically approaching its limit for the micro-quantitative part of variation.” Also, reasons are given why the law is relevant for heterozygotes and allogamous species as well, and the genetical basis of the law is briefly defined.
In addition, arguments are presented why the overoptimism and euphoria at the beginnings of the period of mutation breeding are to be evaluated in connection with the basic assumptions of the synthetic theory of evolution − i.e. the assurance that mutations and selection constitute the entirely sufficient explanation of the origin of all species and higher systematic categories of the plant and animal kingdoms alike. This point established, the question is discussed whether the finite nature of the mutant spectra found in plant breeding research might also have repercussions on the present theory of the origin of species.
Providing an affirmative answer of the applicability of the law of recurrent variation not only to cultivated plant and animal lines but also to species in the wild, the statements and assertions of the synthetic theory as quoted below will have to be revised.
Introduction:
Hermann J. Muller, founder of mutation genetics and winner of the “Nobel Prize in Physiology or Medicine” in 1946, summed up the broad range of aspects and implications of mutation research in his Nobel Lecture on “The Production of Mutations” (71). Among several further topics, he discussed the question of teleological tendencies in the process of variation itself (which he emphatically denied), the proportions of lethal mutations to invisible and to visible ones, also chromosome breakage and heterosis. One key point on mutations in general certainly was his inference that due to the fact that “the great majority of the changes should be harmful in their effects, just as any alterations made blindly in a complicated apparatus are usually detrimental to its proper functioning, many of the larger changes should even be totally incompatible with the functioning of the whole, or, as we say, lethal”. Hence, concerning medical aspects he later emphasized that “it becomes an obligation for radiologists - though one far too little observed as yet in most countries - to insist that the simple precautions are taken which are necessary for shielding the gonads, whenever people are exposed to such radiation, either in industry or in medical practice”. Nevertheless, he was convinced that especially the ‘invisible mutations’ (see below) were the basis of the origin of all life forms, including man.
The pros and cons of two of the key elements of mutation research, which Muller himself had regularly stressed before and continued to do so after his Nobel Lecture, will be investigated in the present paper: (a) the significance of this subject-matter for the synthetic theory of evolution and (b), its applicability to animal and plant breeding. For Muller, there was a clear connection between these two topics, since his confident view of the potentials of mutations for evolution provided the basis for the anticipated utilization in animal and plant breeding. In fact, he was convinced “that for the first time he had willfully changed the hereditary material and that evolution could then be speeded up” (42). Thus, applying this evolutionary hypothesis to breeding research, Muller himself stated that “for the practical breeder, it is hoped that the method will ultimately prove useful” (70), and likewise, that the production of mutations “may...prove of increasing practical use in plant and animal improvement, in the service of man” (71). Although for adaptation he favoured mutations “with effects too small to have been detected at all by our rather crude methods”, he thought that “under conditions of artificial breeding larger mutations also can be nursed through to the point where they become suitably buffered" − referring to Huxley’s idea on the method how larger mutations could possibly be established in populations.
Also, in the accompanying Nobel Presentation Speech by Professor T. Caspersson, member of the Staff of Professors of the Royal Caroline Institute, this idea was presented as follows (16):
“The extended knowledge of the mechanism of the mutation processes has influenced and stimulated the work in numerous fields outside theoretical genetics, and both theoretically and practically important results have been reached. Merely to exemplify the diversity and the varied nature of the spheres touched upon, I beg to adduce a few examples: applied genetics, especially plant improvement, which is of such practical importance, the theory of evolution, metabolic research, a number of spheres within the realm of medicine, especially perhaps eugenics and the theory of disease in general.”
And Muller himself further stated the importance of mutations for plant breeding and evolution in his Nobel lecture as follows (71):
“Not only is this accumulation of many rare, mainly tiny changes the chief means of artificial animal and plant improvement, but it is, even more, the way in which natural evolution has occurred, under the guidance of natural selection. Thus the Darwinian theory becomes implemented, and freed from the accretions of directed variation and of Lamarckism that once encumbered it.”
These statements of Muller and Caspersson should also be seen on the background of a more general revolution in biology: the initiation of the synthetic theory of evolution (neo-Darwinism), launched in 1937 by Dobzhansky’s book Genetics and the Origin of Species (22, 48). From about that time on, the majority of evolutionists viewed mutations (as defined by Muller) and selection to be the fully sufficient driving force of the origin of all species and higher systematic categories of the plant and animal kingdoms alike.
The essential part mutations play in the synthetic theory was later summarized by Jacques Monod in the following plain terms (69):
“We call these events [mutations] accidental; we say they are random occurrences. And since they constitute the only possible source of modification in the genetic text, itself the sole repository of the organism's hereditary structures, it necessarily follows that chance alone is at the source of every innovation, of all creation in the biosphere. Pure chance, absolutely free but blind, at the very root of the stupendous edifice of evolution: this central concept of modern biology is no longer one among other possible or even conceivable hypotheses. It is today the sole conceivable hypothesis, the only one that squares with observed and tested fact. And nothing warrants the supposition - or the hope - that on this score our position is likely ever to be revised.”
The doyen of the synthetic theory, Ernst Mayr, was of the widely accepted opinion that on the basis of mutations, “every aspect of the "wonderful design" so admired by the natural theologians could be explained by natural selection.” (63)
And Richard Dawkins, perhaps the most outspoken contemporary protagonist of the theory, wholeheartedly assents in the following words (18):
“Never were so many facts explained by so few assumptions [mutations and selection]. Not only does the Darwinian theory command superabundant power to explain. Its economy in doing so [by mutations and selection] has a sinewy elegance, a poetic beauty that outclasses even the most haunting of the world's origin myths.”
For a documentation of many further voices principally agreeing with these statements, see 17, 43, 44, 48, 62-64.
Expectations in mutation breeding
Since the origin of cultivated lines was thought to be indispensably due to the same factors as the origin of species in the wild, it has been reported that an enormous euphoria spread among biologists in general and geneticists and breeders in particular that the time had come to revolutionize the "old" method of recombination breeding by the entirely new branch of mutation breeding (see documentation below).
In other words: provided that mutations had, in fact, produced the raw materials for the origin of all genes and proteins, all physiological processes and anatomical structures of both the animal and plant kingdoms alike, the most surprising successes had to be expected by applying these factors − induced mutations and selection − to animal and plant breeding research.
Also, three different time-lapse methods complementing each other for a complete success in a rather short period of time were at the disposal of the breeders: (a) multiplication of mutation rates, (b) well-aimed recombination and (c) intelligent selection. Thus, in the USA as well as in several countries of Europe and Asia, the new research branch of mutation breeding was launched in what might be called two waves: the first billow at the end of the 1930s, which was reinforced especially after the Second World War to form a tide in cooperation with the FAO/ IAEA, worldwide.
Mutation breeding some 40 years later
In the following paragraphs we will condense the general results for mutation breeding after several decades of intense research of this branch by directly quoting the authoritative statements of some of the world’s best agronomical and botanical scientists, most of which have actively taken part in mutation breeding themselves.
Thus, some 40 years after its beginnings Simmonds sums up the inclusive results of the enterprise of mutation breeding in his book on the Principles of Crop Improvement (84):
“Earlier overoptimism, to the effect that induced mutations were about to revolutionize plant breeding, has given place to a more sober appreciation of the technique as a valuable supplement to more conventional techniques in certain, rather restricted circumstances. ....[V]ery many programmes failed, especially in the early days of overoptimism, to produce anything useful because they were not fulfilled. Nowadays we see mutation-induction simply as one technique which is occasionally useful in enlarging the genetic base of a programme in a limited and highly specific fashion.”
Additionally, Leibenguth describes the overall results of mutation breeding in his work Züchtungsgenetik (Genetics of Breeding) as follows (40):
“Almost all mutants distinguish themselves by negative selection values. According to observations in cereals and legumes the proportion of mutants being suitable for breeding amounted to 0.5 to 1 percent of the genotypes selected in these experiments. Besides, often a negative effect on other components of the pleiotropic spectrum of characters has been found that diminishes the breeding value of a positive mutant. Thus, nowadays the original aim to substitute the time-consuming and expensive methods of recombination breeding by “mutation breeding” is not up-to-date anymore. Mutation breedings is viewed to be less an autonomous method of breeding than an occasionally used supplement to traditional methods.”
Already some years before, Micke had stated that “one has to accept the fact that only a very small fraction of induced mutants (certainly less than 1 %) has ever been found suitable to enter yield trials and eventually only 1 % of those evaluated passed the official tests and obtained approval for commercial utilization” (67).
Over and above, Leibenguth adds that mutation breeding cannot be successfully applied to animal husbandry at all, because, “In contrast to plants, animals are genetically more severely balanced. Hence, all kinds of mutations are even more frequently lethal and more strongly diminishing vitality and fertility in animals” (40). Hence, according to all the evidence achieved so far by experimental investigations (and later also by careful considerations in theoretical genetics) there is absolutely no future for mutation breeding in animals − not to speak of severe ethical problems involved in the artificial mutagenesis of birds, mammals and other animals capable of feeling pain.
In plant breeding less than 1 percent of all the induced mutants have been chosen as possible candidates for further investigations. Of these again only 0.5 to 1 percent have passed the necessary further field trials until they were found suitable for commercial use. Thus, in plant breeding the average proportion of negative or useless mutants to positive ones is smaller than 10,000 : 1. Making calculations on the basis that only 0.5 percent of all induced mutation were suitable for further investigations and that again only 0.5 percent displayed a positive selection value for the breeder, this proportion is about 40,000 : 1. An approximate mean value of 25,000 negative (or useless) mutants to 1 being positive should therefore not to be an unrealistic calculation for plant breeding.
As to the genetically even more severely balanced animals, the state of affairs has been so arduous that no realistic numbers have been produced, which could provide the basis of similarly approximate calculations regarding the proportions of negative (or useless) mutants to positive ones in animal husbandry. If − as an educated guess − one multiplies the proportionate number of disadvantageous mutations by the factor of 10, the result would already be some 100,000 to 400,000 negative (or unavailing or neutral) mutants to 1 useful for breeding research.
It was on the basis of such experiences often made over dozens of years that almost all commercial breeding stations in the USA and Europe have deleted mutation breeding from their research programmes.
A significant concrete example may back up this point: at the end of the 1960s it was still widely believed that it was possible to improve crop proteins by mutation breeding. After some one and a half decades of intensive efforts and extraordinary financial input, Micke and Weindl comment (68):
“Our programme on the improvement of grain protein has now come to an end. ...[D]uring the 14 years of the programme it had to be recognized that the matter is more complicated and that there are some mutual limitations of quantity and quality.”
Poehlmann has summed up the overall results of mutation breeding in agreement with the authors quoted above as follows (76):
“One can only conclude that the results from mutation breeding in varietal development of the major field crops have been rather meager in relation to the efforts expended.”
Peter von Sengbusch concurs by the following observation (82):
“In spite of an enormous financial expenditure, the attempt to cultivate increasingly productive varieties by irradiation, widely proved to be a failure.”
Also, the distinguished plant breeders Fischbeck, Röbbelen and Stutzer are in accord with these statements (25):
“The objectives of practical plant breeding, to achieve new opportunities of a gradual and continuous amelioration of tried and tested breeding varieties could...not be realized.”
And especially concerning the neo-Darwinian concept of “micro-mutations” these three authors continue (25):
“Also, the modified concept of a direct use of so-called “micro-mutations” remained unsuccessful, because achievable breeding progress by this method distinctly lagged behind useful variation, which could be developed from the broad stream of conventional recombination breeding.”
Yet, perhaps one of the most astounding facts in the history of genetics appears to be the enormous gulf between the optimistic descriptions of mutants by so many authors active in plant breeding research during that period of time and the later "widely spread disappointment regarding mutation breeding" (66) due to the disconcerting reality, i.e. the meagre results obtained. Confirming the observation of a rather strange distance between hypotheses and reality, Micke continues his assessment after his calculations quoted above (explaining the relatively few useful mutants achieved in mutation breeding) as follows (67):
“In contrast to such rare achievements there have been innumerable 'promising mutants' reported in innumerable publications, which never seem to appear again on the stage after their first presentation. Nevertheless, there remains a respectable number of mutants which even the self-critical breeder or geneticist have seriously considered as progressive and of which only very few so far have contributed to the development of better crop cultivars.
This experience has been disappointing to many, to those who worked with mutations and expected optimistically fast 'break-throughs' as also to those who watched the many mutation activities sceptically but nevertheless hoped that results would make the difficult task of plant breeders easier, at least in particular areas.”
Micke also pointed out that neither the application of different mutagenic agents, nor various degrees of dosages, nor diverse modifying measures were able to revise the overall results: "The ultimate hope of obtaining more of the 'better' mutants has not been fulfilled" (67) (see also note 1 at the end of the paper).
Synopsis
According to the premises of the synthetic theory, explaining the origin of the entire world of organisms predominantly by selected mutations, a worldwide revolution in plant breeding research had been expected in the late 1930s, which was reinforced by Nobel laureate Josef H. Muller in 1946 especially for first decades after the Second World War.
However, due to the fact that:
(a) “many programmes
failed...to produce anything useful”,
(b) “almost all mutants
distinguish themselves by negative selection values”,
(c) “all
kinds of mutations are even more frequently lethal and more strongly
diminishing vitality and fertility in animals”,
(d) the overall
results “have been rather meager in relation to the efforts expended”,
(e) “in spite of an enormous financial expenditure... [mutation
breeding] widely proved to be a failure”,
(f) “the objective
of practical plant breeding...could not be realized” neither by “macro-mutations” nor
by “micro-mutations”,
(g) none of the modifying measures
applied could help fulfilling “the ultimate hope of obtaining more
of the ‘better’ mutants”,
- the overall result was that these strong anticipations concerning a revolution in plant breeding, accompanied by an intense euphoria especially among geneticists and agronomical scientists after the Second World War, ended up in a worldwide failure and breakdown of mutation breeding as an autonomous branch of breeding research in the 1980s at the latest in most Western countries.
The status of mutation breeding today is that of “an occasionally used supplement to traditional methods”, just “occasionally useful in enlarging the genetic base of a programme in a limited and highly specific fashion”.
To answer the question, what this “limited and highly specific fashion’ could essentially consist of, one should be aware of the fact, that mutations usually produce weaker or non-functional alleles of wild-type genes. Such mutagenic effects can be useful in plant breeding research when, for example, some of a plant’s secondary metabolites are disadvantageous for human consumption. If the gene functions necessary to produce such metabolites can be switched off by mutations without greater pleiotropic shortcomings for the plant as a whole, such a mutant could be interesting for further breeding.
In fact, Reinhold von Sengbusch (the father of Peter), perhaps Germany’s most successful plant breeder of the 20th century, summed up the essence of plant breeding by stating that − apart from polyploidy − the transformation from the wild to the cultivated plant is genetically characterized mainly by the fact that the features of the wild plants are dominant and those of the cultivated lines are recessive (83). Usually, recessiveness means losses of gene functions (for a documentation, see 43). As inactivations are the most common effect, which ‘normal’ mutations and/or mutations generated by transposons are exerting on genes (thus producing recessive alleles), the inference can be made that − as far as gene inactivations are important for breeding − mutations might “occasionally” still be relevant for some further progress (see also some reviews on transposons, where these points are further discussed: 1, 2, 10, 11, 38, 43, 54 - 56).
Yet, in our age of molecular genetics, tools are being developed that should increasingly allow directed mutagenesis to inactivate genes coding for undesirable second plant metabolites thus substituting conventional mutation breeding by accidental mutations probably entirely in the near future.
Notwithstanding, the enormous efforts of mutation breeding had also some unequivocally positive side effects for plant breeding research in particular and basic research of biology in general, which will be the topic of our next paragraphs.
Some positive “side effects” of mutation breeding
Although the enormous successes and world-wide revolution firmly expected in plant and animal breeding in connection with the assumptions of the synthetic theory did not materialize, science nevertheless profited from the intense efforts of mutation breeding “by a rapid increase of the information on the localization of genetic effects in the genome of important cultivated plants” (25).
Thus, basic scientific research has substantially benefited from this enterprise. In other words: “Although the production of plant mutants was economically unprofitable, it probably proved to be the most effective experimental instrument of modern basic research” (82).
I have called the most important result of this branch of basic scientific research fully relevant for both, the origin of species and for mutation breeding, the law of recurrent variation (45) (see also note 2 at the end of the paper), which will be the topic of the next paragraphs.
Deducing the law of recurrrent variation
An essential experimental discovery supporting the improbability of the origin of all life forms due to mutation, recombination and selection alone is the fact (well-known for decades) that − after repetitive mutagenic treatment of all the lines and species tested so far − the spectrum of mutants will only slightly be increased. In other words, there is a regularity in the appearance of the overall mutant phenotypes. After 40 years of intensive mutation research in Antirrhinum no less a geneticist than Hans Stubbe has summed up his studies as follows (87):
“The continually improved knowledge of mutants in Antirrhinum has provided some essential experience [or results]. During the years each new large mutation trial showed that the number of really new mutants recognized for the first time, was steadily diminishing, so that the majority of the genetic changes was already known.”
Similarly, Werner Gottschalk, another of the world’s leading mutation geneticists stated (28):
“The larger the mutant collections are, the more difficult it is to extend them by new mutation types. Mutants preferentially arise that already exist.”
In other words, the number of mutants with new phenotypes asymptotically approaches a saturation line in persistently large mutation experiments.
The results of mutation breeding in barley achieved by Udda Lundqvist from the breeding station in Svalöf (Sweden) in decades of experiments will clearly illustrate the phenomenon of recurrently appearing mutants. She reported at the end of the 1980s that during the last 50 years about 9,000 barley mutants have been isolated. Including lethal mutants, there were identified at least 100,000 mutants (59).
The following examples of Table 1 of the recurrent appearance of special types of mutants have been compiled from a paper of Lundqvist (58):
Table 1. Examples of repetitive appearance of certain types of barley mutants compiled according to data published by Lundquist.
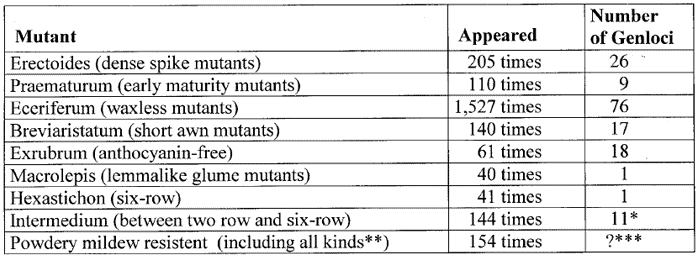
*103 of these cases investigated on 11 int gene loci.
**77 mutants
were resistant against race D1, 48 had complete resistance, and 29 displayed
brown necrosis.
***Of 72 investigated resistant mutants, 54 were found
to be distributed on 8 genes (the 28 recessive mutants belong to one
single locus); for the remaining 18 mutants the number of loci does not
appear to be fully established.
Thus, these 9 types of mutants have appeared altogether 2,422 times. According to Lundqvist the 9,000 barley mutants isolated during some 50 years of extensive mutagenesis experiments, could be grouped into exactly 93 distinguishable types or classes (see Table 2). Many of these mutant types have appeared more than 100 times, some even more than 1,000 times.
Closely comparable results have been achieved for all other crops, which were included in mutation breeding research − as for examples, the pea (Pisum sativum), rice (Oryza sativa), corn (Zea mays), soybeen (Glycine max) and many others (43).
Table 2. Types of mutants in barley (according to Lundqvist (60); numbers in brackets added by W.-E.L).
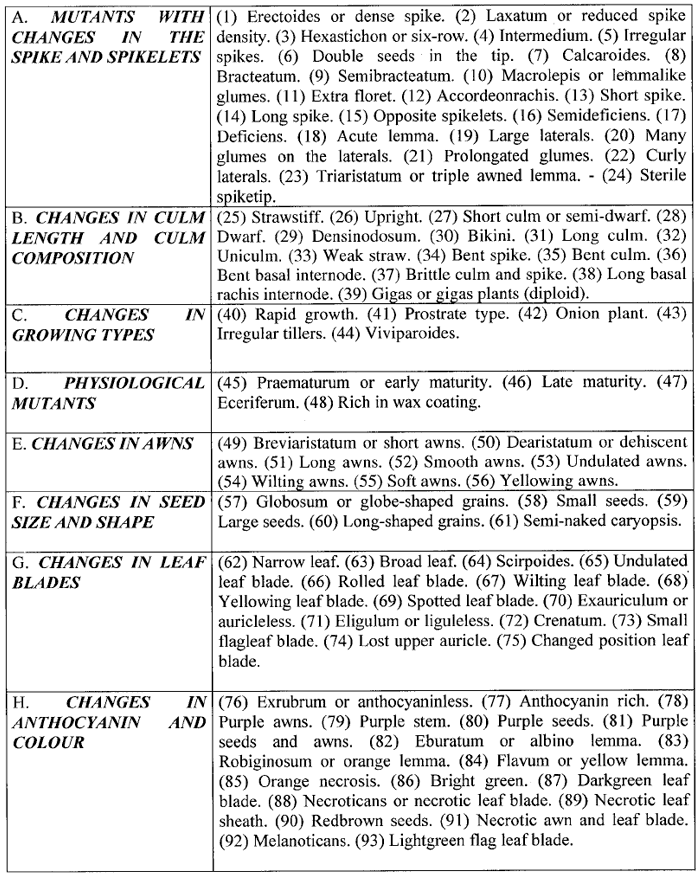
Taking the work of all research stations and breeding companies around the world together, probably millions of barley mutants were induced. Of these only 25 were found to be acceptable for the list of commercial barley cultivars and 33 were added due to recombination (34). However, since the average cultivation time of a commercial barley line is only about 10 years, most of these lines will not be cultivated any more.
Although “mutants for just about every recognizable trait exist” (34), the results in barley may clearly illustrate Poehlmann’s comment, that, as quoted above, “One can only conclude that the results from mutation breeding in varietal development of the major field crops have been rather meager in relation to the efforts expended” (76).
It may also be pointed out in this connection that − as far as the author is aware − neither plant breeders nor geneticists have ever reported the origin of any new species, or just any new stable races or ecotypes either surviving better or at least as well in the wild in comparison with the wild-type, in which the mutation(s) have been induced (43, 45, 52, 55).
As to the recurrently appearing mutants, two pioneers of plant breeding research, Kuckuck and Mudra, emphasized the following key point already in the midst of the last century (37):
“As extensive experiments have shown especially in barley, the entire array of lines of the world seed bank (Weltsortiment) can be mutatively induced by X-rays. ...In part, these induced mutants proved to be genetically identical with similar lines of the world seed bank. In other cases the same phenotypes are due to different genes; thus in the latter cases so-called heterogeneous groups of features have been detected.”
Similar observations have been reported for several other crop plants as rice and maize. Instead of the regular and perpetual formation of new useful culture varieties, subspecies and species, incessantly the same spectrum of mutants is reproduced, so that after a certain number of experiments the method is hardly useful for plant breeding anymore.
My own studies during the last 40 years (including experiments with Pisum, Antirrhinum, and Misopates - altogether more than 2 million plants investigated) are in full agreement with the results of the authors just cited (38, 43-57, 81).
To comprehend and interpret these observations correctly one must clearly distinguish between the two levels of investigations in genetics: first, the level of the phenotypes, and second, the DNA level. On the latter, the potential of missense and nonsense mutations and other sequence deviations is nearly infinite. However, the spectrum of the resulting different phenotypes is not, because the space of functionally valid sequences within a given system of tightly matching regulatory and target genes and correspondingly co-ordinated functions involved in the formation of the finely balanced whole of an organism, cannot infinitely be stretched by chance mutations.
To take a rather crude illustration (see also (44) exemplifying Muller’s case of an apparatus as quoted in the introduction): Drop your computer from the desk or take a screwdriver and a hammer, open the casing, shut your eyes and then forcefully operate in the innards! Depending on the number of computers and how often and for how long one proceeds to act this way, one may collect a nearly endless number of non-functional changes. Yet - with much luck - one may also select a few operationally diminished, but nevertheless still working, systems. Thus, one may demolish a computer in a thousand and more different ways by some accidental procedures. However, the resulting still more or less functional states (the functional phenotypes), will be limited. The hope to secure a Pentium V from a 486er by this method would be very bold indeed. - Of course, the situation in biology is more complex than in engineering, because organisms are, for instance, not only passive, but also reactive entities. Nevertheless, limits to selection have repeatedly been found in several areas of biological research.
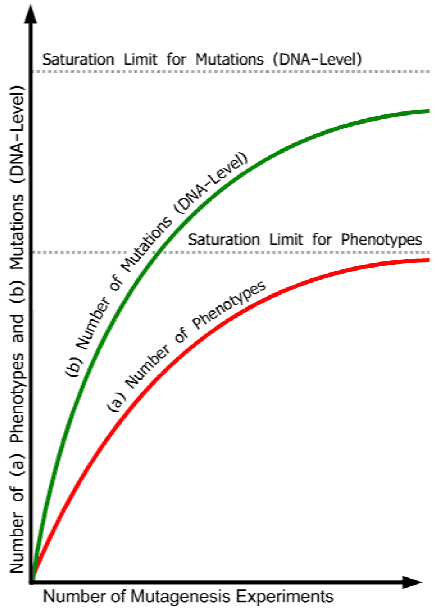
Figure 1. Idealized saturation curves illustrating decrease of the number of new mutant phenotypes and new mutant genes in increased number of experiments until saturation limits are reached. Abscissa: increasing number of mutation experiments. Ordinate: (a) number of mutant phenotypes, and (b) number of mutations (DNA-level) with effects on the phenotype. Because mutations at different loci can cause similar or identical phenotypes (see Table 1 above), the curve for the number of mutant genes is distinct from that of the number of new phenotypes. The redundancy problem - for example, some phenotypes appear only when 2 or more genes have been mutated (see further points in the text) - widens the distance between the two curves. The real curves will be different for different organisms, depending, among other things, on the genetic complexity of the species involved as well as on the scale and specificity of the experiments realized (different kinds and quantities of radiation, chemical mutagens, transposons, t-DNA). The common ground of all curves is the finite number of mutant phenotypes and mutant genes with effects on the phenotype (apart from a micro-quantitative rest of variations due to, for example, environmental and epigenetic factors, position effects, and ‘junk DNA’, which, however, does not change the basic situation).
The aptness of Muller’s comparison of mutations with accidental changes in a complex apparatus has been reinforced by molecular genetics during the last decades. Behe has recently recapitulated the point for a larger audience as follows (6):
“The resemblance of parts of life to engineered mechanisms like a watch is enormously stronger than what Reverend Paley imagined. In the past 50 years modern science has shown that the cell, the very foundation of life, is run by machines made of molecules. There are little molecular trucks in the cell to ferry supplies, little outboard motors to push a cell through liquid.
In 1998 an issue of the journal Cell was devoted to molecular machines, with articles like ''The Cell as a Collection of Protein Machines'' and ''Mechanical Devices of the Spliceosome: Motors, Clocks, Springs and Things.'' Referring to his student days in the 1960's, Bruce Alberts, president of the National Academy of Sciences, wrote that ''the chemistry that makes life possible is much more elaborate and sophisticated than anything we students had ever considered.'' In fact, Dr. Alberts remarked, the entire cell can be viewed as a factory with an elaborate network of interlocking assembly lines, each of which is composed of a set of large protein machines. He emphasized that the term machine was not some fuzzy analogy; it was meant literally.”
Additionally, the observation that none of the different methods of mutagenesis − from delicate experiments looking for optimal mutation frequencies in plant breeding to the most massive mutation inductions − have ever changed the fact of selection limits (detected for all the plant and animal species so far investigated), is in agreement with the facts just mentioned as well as with the saturation curves shown above (43).
One of the best contemporary population geneticists, Daniel L. Hartl, has summed up the question of selection limits as follows (32):
“Progress under artificial selection cannot go on forever, of course. As noted earlier, the population will eventually reach a selection limit, or plateau, after which it will no longer respond to selection. ...However, many experimental populations that have reached a selection limit readily respond to reverse selection.”
And some years later Hartl und Jones have emphasized this empirical fact again (33):
“Population improvement by means of artificial selection cannot continue indefinitely. A population may respond to selection until its mean is many standard deviations different from the mean of the original population, but eventually the population reaches a selection limit at which successive generations show no further improvement.”
Both, in the animal and plant kingdoms, selection limits have been detected, which could not be overcome in spite of persistently intensified mutagenesis. The basic reason is that the spectrum of mutant phenotypes is large but nevertheless limited. Moreover, this gamut of recurrently emerging large yet finite numbers of different mutants is reproducible as many times as correspondingly large populations are repeatedly mutagenized and investigated.
Considering the safe predictability, regularity and reproducibility of the phenomenon, we might thus formulate the law of recurrent variation as shown in the next paragraphs.
Formulating the law of recurrent variation
In agreement with the facts referred to above we can formulate the law of recurrent variation as follows:
Treating homozygous lines with mutagenic agents generates large, but clearly finite, spectra of mutants. This consistently occurs when the experiments are carried out on a scale adequate to isolate the potential of alleles causing phenotypic and functional deviations (saturation mutagenesis). However, due to almost invisible residual effects of changes in redundant sequences and/or of further chromosome rearrangements, the corresponding saturation curve is asymptotically approaching its limit for the micro-quantitative part of variation.
Because mutations at different loci often cause similar or identical phenotypes (most traits are polygenic), the curve asymptotically approaching the saturation line for the mutant genes is distinct from that of the mutant phenotypes. In absolute terms, the distance between the curves is also aggravated by the redundancy of (1) the genetic code, (2) of gene functions, and (3) of conservative amino acid substitutions, allowing mutant proteins to approximately fulfill their original tasks and functions.
However, researchers must constantly remind themselves that the law of recurrent variation focuses only on DNA variations displaying measurable effects on the phenotype and usually disregards the rest (see also transposons, below).
In practice this means that in repetitive mutagenesis experiments the number of new mutants differing phenotypically from each other is steadily diminishing until a saturation limit is reached and progress under artificial selection comes to an end.
Given similar genetical preconditions, the spontaneous mutation process in the wild will produce the same large but limited spectra of mutants, which have appeared in mutagenesis experiments. Yet, due to the decidedly lower mutation rate under natural conditions, much larger populations are needed to realize that potential − apart from the fact that most of the mutants will disappear shortly after their arrival because of their negative selection values.
Also, the law is valid for heterozygous lines and allogamous species. However, due to uninterrupted gene flow the curves will approach the saturation limits more slowly than in homozygous lines and autogamous species.
As for a discussion of the micro-quantitative part of variation, see (45).
The genetic basis of the law of recurrent variation
The genetic reasons for the law are rather simple. There are only a limited number of genes, which, upon mutation, can produce a restricted number of alleles. Stig Blixt has summed up the point from the plant breeder’s view as follows (14):
“The number of gene combinations possible to form from ten thousand genes is infinite. If the plant breeder had to consider the entire gene-material as his field of work, the question posed ["...whether precise controlled plant breeding is a realistic possibility..."] would have to answered in the negative. This, however, is not the case.
A large − probably the major − part of the genes functioning in a plant are most certainly of no concern for the plant breeder, inasmuch as, being essential for the basic function of the cell and differentiation mechanisms, all genetic variation in such genes represent 'forbidden mutations', in the sense that the resulting mutants are completely non-functioning and immediately eliminated. A certain part of the genome may thus exist in probably one specific combination only, representing what may be called the ultra-conservative part of the system. Another large part of the genome, which may then be called the conservative part, seems, although less rigidly, still to be required to be present in the developed form to produce a 'normal' organism able to carry out the plant functions in such a way as to result in a reproductive, competitive individual. Mutations in this part of the genome result in lethals, in sterile individuals, maybe in different kinds of severe chlorophyll deficiencies, and so on.
Thus what has to be dealt with is certainly not the entire genome, but only a part of it, the variable or redundant part, and in all probability this constitutes a minor part. Even if the number of genes may still amount to thousands, this is a restricted and finite number, which will, moreover, also in progenies from crosses, contain homozygous blocks of varying size.”
As to the statement that, “what has to be dealt with, is certainly not the entire genome, but only a part of it, the variable or redundant part, and in all probability this constitutes a minor part” − the question of the biological meaning of transposable elements (TEs) might be raised, since up to about 90 percent of a plant’s genome can consist of different families and classes of TEs. These problems have been extensively discussed in 1, 2, 38, 43, 54-56, 86. If the hypothesis is correct that most TE multiplications constitute weakly parasitic events without doing too much harm for the lines and species thus affected, this relatively huge DNA mass might be largely irrelevant for the plant breeder in the field (there are, however, several pertinent TE-effects, which appear to be similar to normal mutations like the production of alleles, including total inactivations of genes, and possibly some further ones not to be discussed here).
Nonetheless, since the DNA mass (pg) can vary strongly between closely related forms (species of the genus Vicia, for instance, vary between 1.8 and 13.3 pg per haploid genome (72, 74, 75), and because even within the same non-polyploid plant species, the C-value can vary substantially (8, 9) − generally without known serious effects relevant for the plant breeder − we might conclude that the assessment of Blixt quoted above, is essentially still correct for any practical purposes even in our age of molecular genetics (for further details see 1, 2, 10, 38, 55, 56).
So focusing on that variable or redundant part of the genome, in which mutations induce phenotypically deviant, but still rather viable, competitive, and fertile individuals and lines - what kind of variations do we detect upon closer inspection as to quality?
The variations induced are mostly losses-of-function-mutations (often including many alleles with a series of gradually reduced functions) and they are either neutral or slightly disadvantageous for the organisms thus affected, yet in some cases they are useful for the breeder as well as micro-evolution as shown above. Generally, “the great majority of the changes should be harmful in their effects, just as any alterations made blindly in a complicated apparatus are usually detrimental to its proper functioning...” (see Muller, as quoted in the introduction).
However, what has never been achieved by accidental mutations, is the creation of entirely new functional DNA-sequences constituting new genes and new gene reaction chains for novel synorganized anatomical structures and/or physiological functions. Thus, in accord with the laws of probability, examples and cases relativizing the law of recurrent variation have not been observed so far (35, 43, 46, 65, 77, 78, 88, 95, see also note 2). In fact, also all the models and data recently advanced to solve the problem of completely new functional sequences and the origin of new organs and organ systems by random mutations proved to be grossly insufficient upon close inspection and careful scientific examination (for the details, see 12, 13, 27, 36, 41, 73, 90, 91).
Corroboration of the law of recurrent variation by Vavilov’s law of homologous series in variation
On the basis of comparable variations in related species, genera and families in the plant and animal kingdoms, the Russian geneticist and agrobiologist Nikolaj I. Vavilov has formulated the law of homologous series in variation (92, 93). He was able to trace back the beginnings of the law's detection to the opponent of Darwin’s theory, Mivart 1871.
Vavilov has summed up the law as follows (93):
“Species and genera that are genetically closely related are characterised by similar series of heritable variations with such regularity that knowing the series of forms within the limits of one species, we can predict the occurrence of parallel forms in other species and genera. The more closely related the species...in the general system, the more resemblance will there be in the series of variations.”
“Whole families of plants in general are characterized by definite cycles of variability occurring through all genera and species making up the family.”
As to the parallel variations, Haecker (31) commented that they [mostly] appear to be nonessential phenotypic deviations of the species thus affected. Otherwise, he argued, it would be incomprehensible why these variations should be connected with the strongly different types of clearly defined species, genera and families. He also noted that the more widely occurring parallel variations seem to be either due to mutant losses of functions, or display a slightly degenerative to biologically indifferent character without tightly correlative connections to the species as a whole.
The most important inference of Vaviliov's law corroborating the law of recurrent variation consists of the fact that the parallel series of recurrent mutant phenotypes − generally including closely related forms − do not level out the essential species and genera differences. Otherwise it should be possible to mutationally transform one species or genus into another closely related one. However, nobody has ever reported something like the morphological transformation of a pea (Pisum sativum) into a fertile grass pea (Latyrus sativus) (39).
The essential difference between the two laws consists of the fact that, although Vavilov clearly noted and carefully studied the parallel variations, he did not comment on the finite nature of the variations, the clearly defined spectra of mutants themselves, which constitutes the inmost nature or substance of the law of recurrent variation. Moreover, not all variation is parallel variation − especially so in distant plant and animal families and orders. However, in any case, the law of recurrent variation applies as formulated above.
Repercussions for the synthetic theory of evolution
Since the foundations of the earlier “overoptimism” for a revolution in plant breeding due to induced mutations rested on the pillars of the synthetic theory of evolution (neo-Darwinism) − insisting, as pointed out above, that mutations and selection are responsible for the origin of all genes and proteins, all physiological processes and anatomical structures of both the animal and plant kingdoms − the question should be permitted whether the failure of mutation breeding has had any repercussions on neo-Darwinism itself.
As far as the author is aware, the protagonists of the synthetic theory have, so far, avoided an adequate scientific discussion of this problem (45).
Nevertheless, the general non-performance or deficiency of mutation breeding after altogether some 50 years of extensive experiments with billions of induced mutations on several continents might point to a fundamental problem of the present theory of evolution as to the question whether mutations are really as productive as postulated and still generally believed to be.
Moreover, several recent peer-reviewed and further publications have added to the weight of this question on the basis of extensive additional biological facts and mathematical calculations (for the details, see 3-7, 12, 13, 19-21, 30, 35, 43-57, 61, 77, 78, 79, 80, 88, 90, 91, 95). In contrast to the neo-Darwinians quoted in the introduction, the unanimous conclusion of these authors - among them now again several Nobel laureates in their relevant disciplines (Sir John Eccles (23), Karl von Frisch (26), Ragnar Granit (29), Charles Townes (89), Eugene P. Wigner (94), and others, see (50)) - is that mutations (in cooperation with natural selection) constitute only a partial solution to cope with the complex task of the origin of species and higher systematic taxa and thus alternatives must be looked for and carefully considered.
As far as the critical authors are acquainted with plant breeding research and animal husbandry, they argue that the relatively few positive mutation results have been mainly due to losses of undesirable features and functions, for example, lupines free of alkaloids, rapeseed without eruca acid, peas with extended tendrils instead of compound leaves etc. Yet, they emphasize the significant point that such losses of function cannot explain the origin of all the genetic ‘raw materials’ necessary for natural selection to generate the entire world of organisms.
Most of these researchers (including the author of the present paper) have no problem in conceding, however, that mutations and selection, as well as genetic drift, might essentially be involved in microevolution, i.e. the formation of races and subspecies as well as some higher systematic categories as species and genera, which were originated by losses of gene functions, as for example, the many cases of losses of flying abilities in insects and birds on islands around the world, losses of scales in fish species in closed lakes, losses of dispersion systems in island plants, organ losses in cave animals etc.) (38, 43, 55).
Concluding remarks
(1) Since in all cases of sufficiently extensive mutagenesis experiments (saturation mutagenesis) a large, but well-defined spectrum of mutant phenotypes has been realized (asymptotically approaching the saturation line especially for the micro-quantitative part of variation), the possibilities and limits of mutation breeding are directly defined by the areas and boundaries of this spectrum of mutants of the lines treated within a species.
(2) Similar to the possibilities and limits of mutation breeding of a line tested within a species, the genetic boundaries of a species in its entirety are defined by the potentials and limits of the gamut of functional alleles potentially realizable for the redundant part of the genes and genomes of all individuals belonging to an ‘actually or potentially interbreeding natural population, which are reproductively isolated from other such groups’ (or, in other words, the redundant genetical part of the genomes of all recombinants and lines belonging to the “most inclusive reproductive community”, i.e. to a Mendelian population). Although it is self-evident that the genetic potential of an entire species is usually larger than that of only one or a few line(s) within a species, the dormant spectrum of mutant alleles in the redundant part of an entire plant or animal species defines the correspondingly much larger but also clearly finite boundaries of a species.
(3) In accord with the law of recurrent variation, mutants in every species thoroughly examined (from pea to man) − whether naturally occurring, experimentally induced, or accidentally brought about − happen in a large, but nevertheless limited spectrum of phenotypes with either losses of functions or neutral deviations. Yet, in the absence of the generation of new genes and novel gene reaction chains with entirely new functions, mutations cannot transform an original species into an entirely new one. This conclusion agrees with all the experiences and results of mutation research of the 20th century taken together as well as with the laws of probability. Thus, the law of recurrent variation implies that genetically properly defined species have real boundaries that cannot be abolished or transgressed by accidental mutations.
In contrast to the authors quoted in the introduction, yet in accord with the group of researchers referred to under REPERCUSSIONS above, the origin of the world of living organisms must be explained on a basis different from that given by the synthetic theory of evolution.
For an additional detailed discussion of further points and possible objections, see (see 1-9, 15, 20, 21, 23, 27, 30, 35, 38, 39, 43-57, 61, 65, 77-80, 86, 88, 90, 91, 94, 95)
Acknowledgements
Figure 1 was drawn by the computer specialist Frieder Meis, Ludwigshafen, Germany. Markus Rammerstorfer, Linz, Austria, suggested some further references and changed the numbers accordingly. The English language was checked by Hal Flemings, San Diego, USA.
Notes
(1) Being aware
of the fact that mutation breeding proved to be useless in animals
and remembering the unanimous critical verdict on mutation breeding
in plants by all competent breeders and botanists alike, to wit that “the
results... have been rather meager in relation to the efforts expended” (as
documented above), the question may be raised how to integrate into
these overall conclusions the different impression sometimes elicited
by the FAO/IAEA Mutant Varieties Database (2004), speaking of 2,337 “officially
released varieties” due to induced mutations? Although in absolute
terms this number seems to be impressive at first sight, for a correct
assessment it has to be evaluated on the background of the following
information:
(a) The number includes ornamental plants, where losses
of gene functions (e.g. for leaf- and flower colour variation) are
even more relevant than for crop plants, (b) according to the FAO/IAEA “the
use of these mutants have been mostly local or regional”, (c)
also, the usage of these mutants was mainly temporal (as in most
culture varieties), (d) the higher the test standards and the more
thorough the testing procedures for officially approved varieties
of a country, the less the number of approved varieties due to mutation
breeding (e.g. most European countries have different standards as
compared to several African countries), (e) mutants have not been
consistently distinguished from recombinants (“cross” is
in fact given as one of the mutagenic agents), (f) in obligate outbreeders
the investigator almost always works with heterozygous lines (whether
a line is improved - in relation to the aims of a plant breeder -
by mutation or recombination, can in many cases only be definitely
ascertained by molecular investigations), (g) the problem to distinguish
recombination of given alleles from new mutations might be relevant
to a ceratin extent even in inbreeders due to the fact, that a certain
amount of cross-pollinations usually occurs in them as well (for
example in my own investigations in peas the outbreeding rate was
1-3%, so that up to 30% of the plants proved to display “contaminations”),
a fact often not duly considered in the work of mutation breeding
with such species and lines (interestingly a v.i.p. of the IAEA breeding
department once admitted this situation in a discussion with me,
but was of the opinion that this made no difference to him), (h)
such points as just enumerated may belong to the reasons for the
statement, that “the IAEA does not warrant the safety, quality,
viability ore purety (genetic or mechanical) of the material” for
international exchange, (i) the rest of truly induced mutations useful
for the plant breeder is usually due to losses of gene functions
in accord with the last paragraphs of the SYNOPSIS above, (j) Neither
the DFG (Deutsche Forschungsgemeinschaft/German Research Foundation)
nor the EU (European Union) has supported mutation breeding during
the last decades, (k) Germany’s practical plant breeders have
abandoned mutation breeding in the 1980s at the latest, which applies
for most European Countries, (l) last not least the number given
above must be seen in relation to the overall “officially released
varieties” on the same world-wide scale and over the same time
period, which probably will amount to several hundreds of thousands
of varieties (just to give a few numbers: there are 1,192 officially
acknowledged rose varieties in Germany alone - but the approval is
always given for only 10 years, so that the number of officially
released rose varieties for the last 50 yeras will be higher - ,
there are 3,200 different potato varieties, which are cultivated
in over 100 countries (2003), and there are about 7,500 cultivated
apple varieties.
(2) In biology the term “law” is often interchangeably used with the label “rule”, as in the case of the Mendelian “rules” or “laws”. Strictly speaking, a law makes testable predictions on the basis of a set of preconditions and does not permit any exceptions from its deductions. Since so far I do not know of any valid exceptions of this principle for induced and spontaneous random mutations as deduced above, I presently prefer to speak of the “law” of recurrent variation sensu stricto (researchers should, perhaps, constantly remind themselves that not only mutation breeding but also any expectations to artificially ‘speed up evolution’ (Muller) by mutations in the wild largely failed because of this law). Yet, borderline-cases could possibly consist of complex DNA rearrangements leading to unusual losses of genetic functions and correspondingly rare morphological aberrations with a low probability to independently occur again, e. g. Tunicate in maize (Thomas Münster, MPIZ, Cologne, personal communication), or the polymorphism conveying powdery mildew resistance in barley (Pifanelli et al., Nature 430: 887-891, 2004). I suggest that such cases should be included in the law as rare “borderline-cases” at the more distal part of the saturation curve for phenotypes, inasmuch as the barley example confers a similar phenotype to the other mlo loss of function alleles (apart from the reversion-rate of 0.5-1 x 10-4 and a “low level growth of sporulating Bgh colonies” in the mlo-11 plants). Moreover, it should perhaps also be considered that in both cases further independent occurrences cannot be excluded in the billions of plants grown each year world-wide in both species: the world barley production was some 155 million tons in 2004 consisting of about 3.8 x 1015 individual gains (one grain ca. 0.04 g). Hence, the occurrence of a grain carrying a mutant gene with the low probability of only 10-12 per gene per generation due to a spontaneous mutation will still amount to several thousands of independent occurrences (and thus grains carrying the mutant gene) worldwide. The number of mutant plants will be smaller, of course, but still amounts to several dozens and several hundreds in a hundred years. A similar calculation can be made for the 705 million tons of maize also produced in 2004 (one grain ca. 0.2 g). The law, however, clearly excludes the origin of new complex functional sequences (entirely new genes and new gene reaction chains for novel synorganized anatomical structures and/or physiological functions) by random mutations. As for the probability of the origin of new functional genes see and (19)-(21), (77), (78), and (95).
References
1. Becker, H.-A., and Lönnig, W.-E. 2002, Nature Encyclopedia of Life Sciences, Nature Publishing Group, London, 18, 559-539. 2. Becker, H.-A., Saedler, H., and Lönnig W.-E. 2002, Encyclopedia of Genetics, S. Brenner and J.H.Miller (Eds.) Academic Press, San Diego, 4, 2020-2033. 3. Behe, M.. J. 1996, Darwin’s Black Box, The Free Press, New York. 4. Behe, M.. J.2002, Natural History 111, 74. 5. Behe, M. .J. 2004, Debating Design: From Darwin to DNA, W. A. Dembski, M. Ruse (Eds.), Cambridge University Press, Cambridge, 352-370. 6. Behe, M.. J. 2005, Design for Living. The New York Times. 7 February 2005, Editorial Desk, p. 21. 7. Behe, M. J. and Snoke, D. W. 2004, Simulating evolution by gene duplication of protein features that require multiple amino acid residues. Protein Sci. 13, 2651-2664. 8. Bennett, M. D., and Leitch I.J. 1995, Ann. Bot. 76,113-176.
9. Bennett, M. D., and Leitch I.J. 1997, Ann. Bot. 80,169-196. 10. Bennetzen J.L. 1998, Curr. Opin. Plant. Biol. 1, 103-108.
11. Bennetzen, J.L. 2000, Plant Mol. Biol. 42: 251-269. 12. Berlinski, D. 2003, Commentary 115: 29-37 (4 April 2003). 13. Berlinski, D. 2003, Commentary 115 (for the full discussion see David Berlinski’s articles under: http://www.discovery.org/csc/fellows.php) (8 July 2003) 14. Blixt, S. 1972, Agri Hort. Genet. 30, l-293. 15. Campbell J.A., Meyer. and S. C. (Eds) 2003, Darwinism, Design, and Public Education, Michigan State University Press, East Lansing. 16. Caspersson, T. 1946, The Nobel Prize in Physiology or Medicine 1946 Presentation Speech. From: Nobel Lectures, Physiology or Medicine 1942-1962, Elsevier Publishing Company, Amsterdam. 17. Darwin C.R. 1859, On the Origin of the Species, John Murray, London. 18. Dawkins, R. 1995. River out of Eden. Basic Books, New York. 19. Dembski, W. A. 1998, The Design Inference: Eliminating Chance Through Small Probabilities, Cambridge University Press, Cambridge. 20. Dembski, W.A. 2002, No Free Lunch: Why Specified Complexity Cannot Be Purchased without Intelligence, Rowman & Littlefield, Lanham.
21. Dembski, W.A. 2004, The Design Revolution, InterVarsity Press, Downers Grove. 22. Dobzhansky, T. 1937, Genetics and the Origin of Species, Columbia University Press, New York 23. Eccles, J.C. 1992, Cosmos, Bios, Theos, 160-164. Margenau, H, and Varghese, R. A. (Eds.), Open Court, Chicago and La Salle, Illinois. 24. FAO/IAEA, 2004, http://www-mvd.iaea.org/MVD/default.htm 25. Fischbeck, G., Röbbelen, G., Stutzer, D. 1987, Landwirtschaftliche Pflanzenzüchtung in Deutschland. Herausgegeben vom Bundesverband Deutscher Pflanzenzüchter e.V. Bonn. Gelsenkirchen-Buer. 26. Frisch, K. von, 1977, Scientific Interview, Third TV-Programme, FRG, 15. 9. 1977. 27. Gene, M. 2001, Randomly Generated Biologically Functional Proteins? http://www.idthink.net/biot/ranprot/index.html 28. Gottschalk, W. 1989, Allgemeine Genetik. 3. Auflage, Georg Thieme Verlag Stuttgart. 29. Granit, R. 1992, Cosmos, Bios, Theos, 177-178. Margenau, H, and Varghese, R. A. (Eds.), Open Court, Chicago and La Salle, Illinois. 30. Grasse, P.-P. 1977, Evolution of Living Organism. Academic Press, New York, N.Y.
31. Haecker, V. 1925, Pluripotenzerscheinungen. Gustav Fischer Verlag, Jena. 32. Hartl, D. L. 1988, A Primer in Population Genetics. Second Edition. Sunderland/Mass. 33. Hartl, D. L. and Jones, E. W. 1998, Genetics. Jones & Bartlett Publishing, Sudbury, Boston. 34. Hockett, E. A., and Nilan, R.A.1985, Rasmusson, D.C. (Ed.), Barley. Madison, 187-230. 35. Junker, R. and Scherer, S. 2001, Evolution - Ein kritisches Lehrbuch. Weyel Lehrmittel Verlag, Gießen. 36. Keefe A.D., Szostak J.W. 2001, Nature 410, 715-718. 37. Kuckuck, H. and Mudra, A. 1950, Lehrbuch der allgemeinen Pflanzenzüchtung. Hirzel Verlag, Stuttgart.
38. Kunze, R., Saedler, H., and Lönnig, W.-E. 1997, Adv. Bot. Res. 27, 331-470. 39. Lamprecht, H. 1974, Monographie der Gattung Pisum. Steiermärkische Landesdruckerei, Graz. 40. Leibenguth, F. 1982, Züchtungsgenetik. Gustav Fischer Verlag, Stuttgart.
41. Lenski, R.E., Ofria, C., Pennock, R.T., and Adami, C. 2003, Nature 423, 139-144. 42. Linskens, H.F. 1982, Review of: Genes, Radiation and Society - Book by E.A.Carlson. Theor. Appl. Genet. 63, 200. 43. Lönnig, W.-E. 1993, Artbegriff, Evolution und Schöpfung, Naturwissenschaftlicher Verlag, Köln (and Internet edition 2002). 44. Lönnig, W.-E. 2001, The Corsini Encyclopedia of Psychology and Behavioral Sciences, Craighead, W.E., and Nemeroff. C.B. (Eds), John Wiley & Sons. New York, 1008-1016 45. Lönnig W.-E. 2002, Das Gesetz der rekurrenten Variation, Naturwissenschaftlicher Verlag, Köln. (See also 1995: Streifall Evolution, Mey, J., Schmidt, R., Zibulla, S. (Eds.), 149-169.) 46. Lönnig W.-E. 2002, Evolution durch Genduplikationen? Naturwissenschaftlicher Verlag, Köln. 47. Lönnig, W.-E. 2002, Coryanthes und Catasetum, Naturwissenschaftlicher Verlag, Köln. 48. Lönnig, W.-E. 2003, Johann Gregor Mendel: Why his discoveries were ignored for 35 (72) years, Naturwissenschaftlicher Verlag, Köln. 49. Lönnig, W.-E. 2004, Dynamical Genetics. Parisi, V., De Fonzo, V., Aluffi-Pentini, F. (Eds). Research Signpost, Trivandrum, India. 50. Lönnig, W.-E. 2005, Nobelpreisträger pro Intelligent Design und/oder einen "religious impulse" in den Naturwissenschaften. Naturwissenschaftlicher Verlag, Köln.
51. Lönnig, W.-E., and Becker, H.-A. 2004, Nature Encyclopedia of Life Sciences, Nature Publishing Group, London. 52. Lönnig, W.-E., and Becker, H.-A. 2004, The Concise Corsini Encyclopedia of Psychology and Behavioral Science, Craighead, W.E., and Nemeroff. C.B. (Eds), Wiley-VHC, New York, Vol. 3, 1008-1016. 53. Lönnig, W.-E., and Huijser, P. 1994, Plant molecular biology manual. Gelvin, S. B., and Schilperoort, R. A. (Eds.), Kluver Academic Publishers, Dordrecht, K1, 1-15. 54. Lönnig, W.-E., and Saedler, H. 1994, Mol. Gen. Genet. 245, 636-643. 55. Lönnig, W.-E., and Saedler, H. 1997, Gene 205, 245-253. 56. Lönnig, W.-E., and Saedler, H. 2002, Annu Rev. Genet. 36, 389-410 57. Lönnig, W.-E., Saedler, H., Kim, J.H. 2005 (in press). 58. Lundqvist, U. 1986, Svalöf 1886-1986, Research and Results in Plant Breeding. G. Olsson (Ed.), Stockholm, 76-84. 59. Lundqvist, U. Personal communication 1987. 60. Lundqvist, U. Personal communication; letter including all the details 1988.
61. Margulis, L., Sagan, D. 1997, Slanted Truths: Essays on Gaia, Symbiosis, and Evolution. Springer-Verlag, Berlin. 62. Mayr, E. 1970, Populations, Species, and Evolution, The Belknap Press of Harvard University Press, Cambridge, Mass. 63. Mayr, E. 2000, Scie. Am., 283 (July 2000), 66-71. 64. Mayr, E. 2002, Laborjournal 5/2002, 26-30.
65. Meyer, S. C. 2004, Proc. Biol. Soc. Washington 117, 213-239. 66. Micke, A. 1970, Genetica Agraria 22, 262-268. 67. Micke, A. 1976, Induced mutations in cross-breeding. IAEA (International Atomic Energy Agency), 1 - 4. Wien. 68. Micke, A. and Weindl, K. 1983, Mutation Breeding Newsletter 2l , 1. 69. Monod, J. 1971, Chance and Necessity. An Essay on the Natural Philosophy of Modern Biology. Knopf. New York. 70. Muller, H .J. 1928, Science 66, 84-87.
71. Muller, H .J. 1946, The production of mutations, Nobel Lecture. From: Nobel Lectures, Physiology or Medicine 1942-1962, Elsevier Publishing Company, Amsterdam. 72. Murray, M.G., Peters, D.L., Thompson, W.F. 1981, J. Mol. Evol. 17, 31-42. 73. Nilsson, D.E., Pilger, S. 1994, Proc. Roy. Soc. London B 256, 53-58. 74. Nouzová, M., Kubaláková, M., Zelová, M.D., Koblítzková, A., Neumann, P., Dolezel, J., Macas, J. 1999, Ann. Bot. 83, 535-541. 75. Nouzová, M., Neumann, P., Navrátilová, A., Galbraith, D.W., Macas, J. 2000, Plant Mol.Biol. 45, 229-244. 76. Poehlmann, J. M. 1987, Breeding Field Crops. Third Edition. Van Nostrand Reinhold. New York. 77. ReMine, W.J. 1993, The Biotic Message, St. Paul Science Publishers, Saint Paul. 78. Scherer, S. Typen des Lebens. Pascal Verlag, Berlin. 79. Schwabe, C. 2001, The Genomic Potential Hypothesis: A Chemist’s View of the Origins, Evolution and Unfolding of Life. Landes Bioscience. Georgetown, Texas. 80. Schwabe, C. 2002, Anat. Rec. 268, 171-179.
81. Schwarz-Sommer, Z., de Andrade Silva, E., Berndtgen , R., Lönnig, W.-E., Müller, A., Nindl, I., Stüber, K., Wunder, J., Saedler, H., Gübitz, T., Borking, A., Golz, J.F.; Ritter, E., Hudson A. 2003, Genetics 163: 699-710. 82. Sengbusch, P. von 1989, Botanik. Blackwell, Hamburg. 83. Sengbusch, R. von 1980, Von der Wildplanze zur Kulturpflanze. Published by author (no place of publication given). 84. Simmonds, N.W. 1979, Principles of Crop Improvement, Longman, London. 85. Simmonds, N.W., and Smartt, J. 1999, Principles of Crop Improvement, Second edition, Blackwell Publishing, Oxford. 86. Sternberg, R. v. 2002, Ann. N.Y. Acad. Sci. 981, 154-188. 87. Stubbe, H. 1966, Genetik und Zytologie von Antirrhinum L. sect. Antirrhinum. VEB Gustav Fischer Verlag, Jena. 88. Swift, D. 2002, Evolution under the Microscope, Leighton Academic Press, Stirling University Innovation Park. 89. Townes, C. H. 1992, Cosmos, Bios, Theos, 122-124. Margenau, H, and Varghese, R. A. (Eds.), Open Court, Chicago and La Salle, Illinois. 90. Truman, R. 2004, Evaluation of neo-Darwinian Theory with Avida Simualtions? PCID 3.1.1. part 1. http://www.iscid.org/papers/Truman_ComplexFeatures1_070104.pdf
91. Truman, R. 2004, Evaluation of neo-Darwinian Theory with Avida Simualtions? PCID 3.1.1. part 2. http://www.iscid.org/papers/Truman_ComplexFeatures2_070104.pdf 92. Vavilov, N. I. 1922, J. Genet. 12, 47-89. 93. Vavilov, N. I. 1935, Chron. Bot. 13, 55-94.
94. Wigner, E. P. 1992, Cosmos, Bios, Theos, 131-132, 270-278. Margenau, H, and Varghese, R. A. (Eds.), Open Court, Chicago and La Salle, Illinois. 95. Wittlich, K. 1991, Über die Wahrscheinlichkeit der zufälligen Entstehung brauchbarer DNA-Ketten. In: Zehn Paradebeispiele gegen Zufalls-Evolution (W.-E. Lönnig, ed.), 28-39, Naturwissenschaftlicher Verlag Köln, Cologne.
The text was slighty corrected on 1 Dezember 2007.
| Zurück zur Homepage |